If NIA's President Gerard Adams discovers another high quality small-cap psychedelic stock that is likely to become his next Numinus Wellness (TSXV: NUMI) or Revive Therapeutics (CSE: RVV) he will be announcing it exclusively to members of www.psychstocks.com. Right now, Psychstocks is free to join, but Gerard will begin charging new members a monthly membership fee on January 1, 2021. Lock in your lifetime FREE membership today at: www.psychstocks.com
On Saturday, December 5th, NIA issued an important Numinus Wellness (TSXV: NUMI) alert at $0.78 per share in which we explained the significance of NUMI's collaboration with MAPS for a compassionate access trial in Canada of MDMA-assisted psychotherapy for the treatment of PTSD. Amazingly, the news initially got almost no reaction when it was released on December 2nd, because of the fact that MAPS is a non-profit organization that the average bandwagon investor in the psychedelic space never even heard of prior to the announcement.
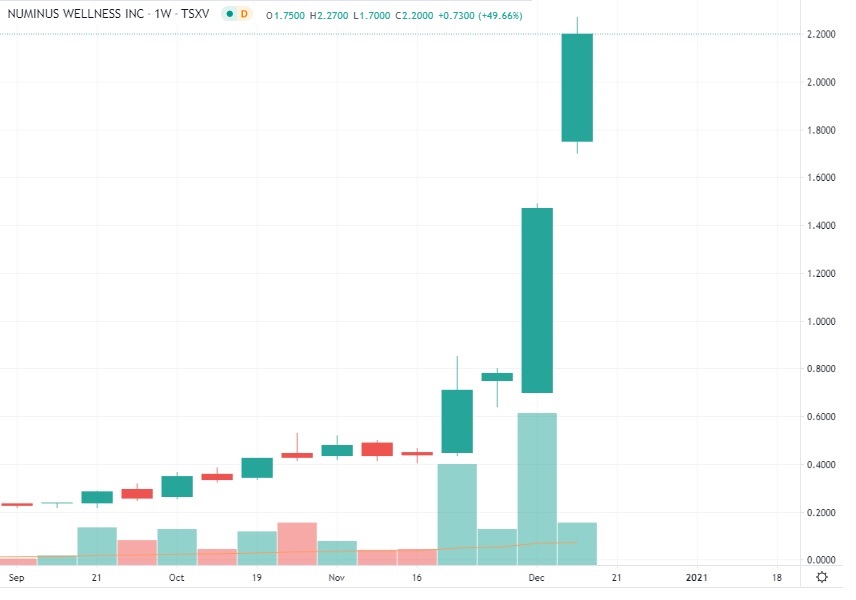
After NIA sent out the news on Saturday, December 5th and explained its significance, NUMI gained by 88.46% last week to close Friday at $1.47 per share. NUMI gained 9X more last week than it did during the prior week when NUMI's MAPS news was first published. This past weekend, the news began to spread among the rest of the investment community. NUMI has rallied another 54.42% this morning to hit a new all-time high of $2.27 per share for a gain of 696.49% since NIA's September 27th suggestion of NUMI as its #1 favorite psychedelic stock suggestion and one of NIA's Top 5 favorite overall stock suggestions at $0.285 per share. NUMI's U.S. OTC symbol LKYSF traded record volume on Monday, September 28th, the first trading day following NIA's initial NUMI announcement.
NIA expects MAPS to receive FDA approval of MDMA-assisted psychotherapy for the treatment of PTSD as early as 2022 with MDMA-assisted psychotherapy likely to become available for PTSD sufferers (victims of the military industrial complex and its endless wars) as early as 2023. We expect MAPS to receive FDA approval of MDMA-assisted psychotherapy prior to Compass Pathways (CMPS) receiving FDA approval of psilocybin-assisted psychotherapy for treatment-resistant depression.
As MAPS Founder Dr. Rick Doblin explained to NIA's President Gerard Adams last year, "We're basically gonna have six years of being the monopoly seller of MDMA. There's 10 million people in the U.S. that have PTSD. We're talking about an incredible need for the product."
Besides NUMI's proposed compassionate access trial in Canada in collaboration with MAPS of MDMA-assisted psychotherapy for the treatment of PTSD, the most significant psychedelic clinical trial of a small-cap publicly traded company in North America is Revive Therapeutics (CSE: RVV)'s proposed "Phase I Study of the Safety and Feasibility of Psilocybin in Adults with Methamphetamine Use Disorder."
RVV has already proven its ability to take a drug called Bucillamine into Phase III FDA clinical trials and they have a strong clinical trial partner the University of Wisconsin School of Medicine and Public Health that RVV has already signed a Clinical Trial Agreement with! RVV's clinical trial partner is already conducting a similar study of psilocybin as a treatment for Opioid Use Disorder, but we believe RVV has made the best choice to focus on Methamphetamine Use Disorder because it has no FDA approved treatments, but psilocybin is already showing tremendous promise!
Past performance is not an indicator of future returns. NIA is not an investment advisor and does not provide investment advice. Always do your own research and make your own investment decisions. This message is not a solicitation or recommendation to buy, sell, or hold securities. NIA has received compensation from NUMI of USD$30,000 cash for a six-month marketing contract. NIA has received compensation from RVV of USD$30,000 cash for a six-month marketing contract. This message is meant for informational and educational purposes only and does not provide investment advice.