NIA Sunday Evening Update: Must Read Immediately
In NIA's opinion, the only investment better than owning a massive resource of gold and/or…
Highlander Silver NYSE Trading Begins on Wednesday
Highlander Silver (TSX: HSLV) will list on the NYSE American Exchange beginning on Wednesday under…
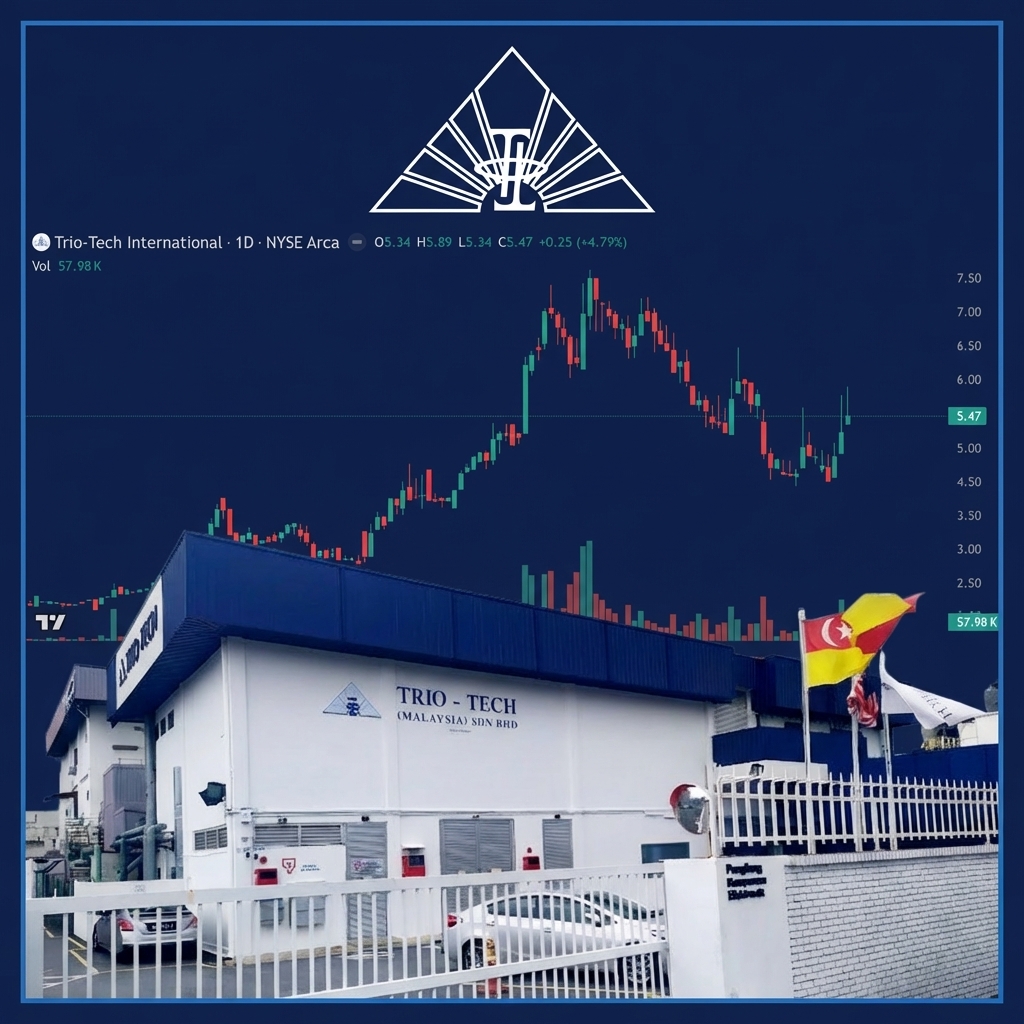
Will TRT Be $50+ Within Months?
Trio-Tech International (TRT) gained for its third straight day on Friday surging 4.79% to $5.47…
Natural Hydrogen = Bitcoin in 2015
Yesterday morning, NIA explained that Trio-Tech International (TRT) is the safest stock that NIA has…
TRT: Possible Rivian R2 Connection
If you read Trio-Tech International (TRT)'s press release from Wednesday, we know that TRT developed…
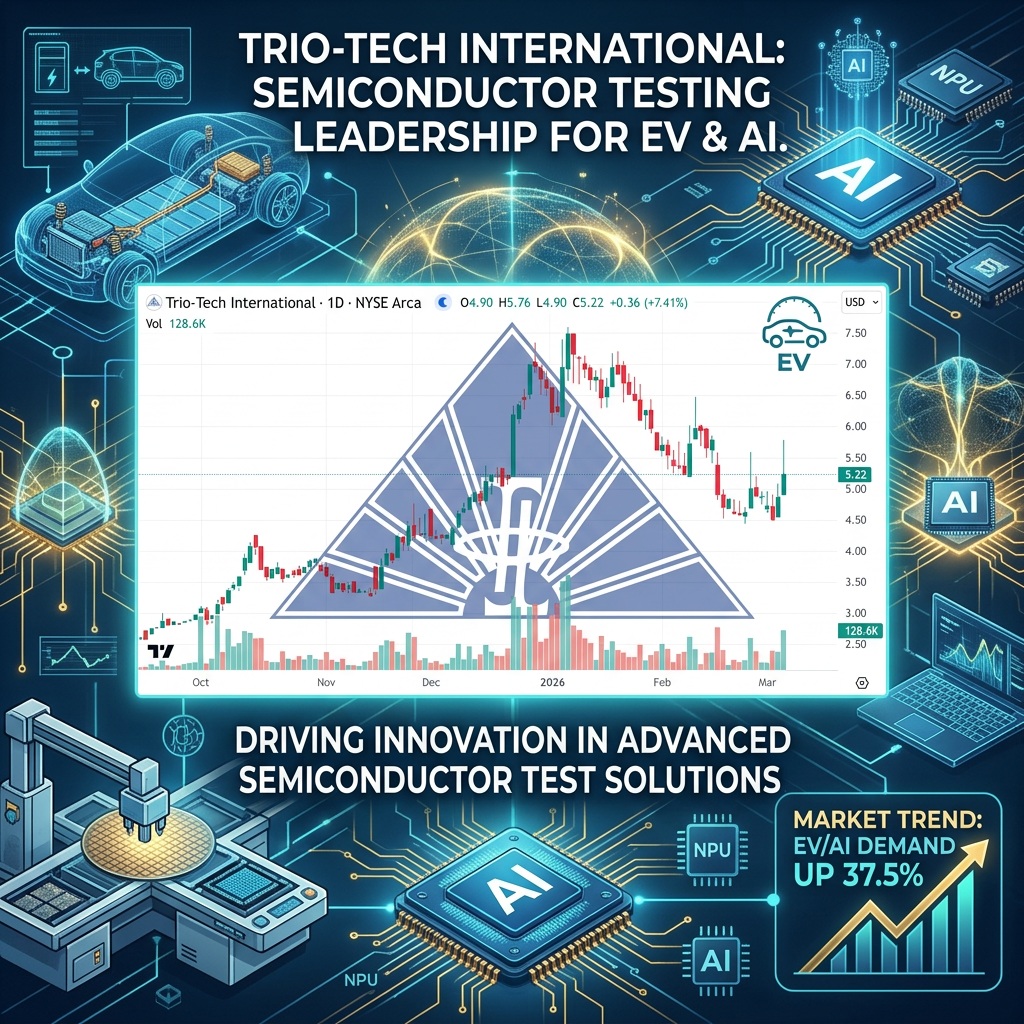
TRT Gains 7.41% to $5.22 and Has 100% Chance of Going Much Higher
Trio-Tech International (TRT) gained by 7.41% today to $5.22 per share and has a 100%…
The Safest Stocks in Today’s Market
In NIA's opinion, Trio-Tech International (TRT) is the safest stock in the market with a…
Going “All-In” on Trio-Tech International (TRT)?
We currently have the biggest stock market bubble in world history, and Trio-Tech International (TRT)…
NIA’s #2 Overall Pick Wins $2.5M Automotive Semiconductor Order
Trio-Tech International Secures Production Burn-In Order from a Leading Integrated Device Manufacturer, Strengthening its Automotive…
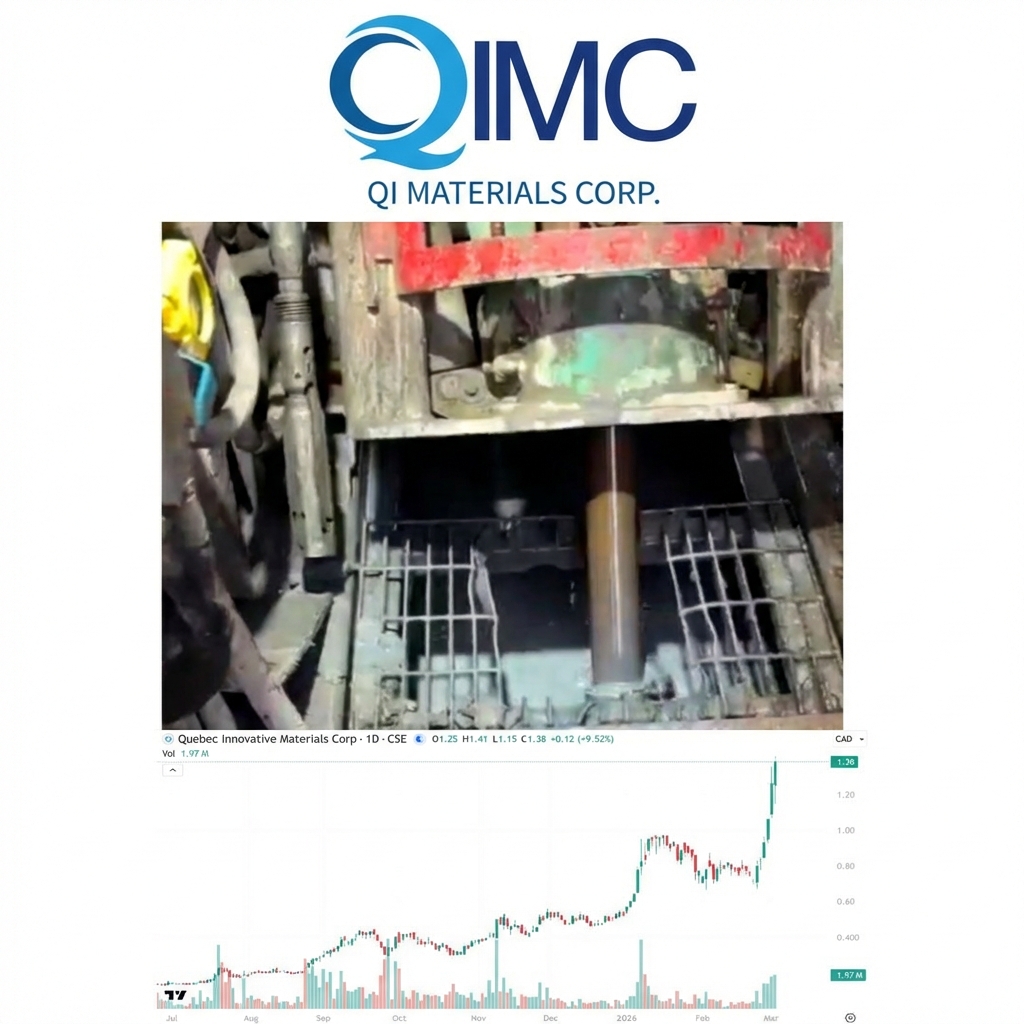
QIMC Soars 9.52% to New All-Time High of $1.38, Intersects Third Hydrogen-Bearing Structural Zone
QI Materials (CSE: QIMC) is the biggest discovery of our careers and surged by 9.52%…